
Best Quality with Disposable Parts IV Infusion Set with Regulator
Anping County Dinghe Wire Mesh Co., Ltd.- Type:Infusion Set
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Five Years
- Group:Adult
- Logo Printing:Without Logo Printing
Base Info
- Model NO.:qk-11
- Transport Package:PE
- Specification:61*38*32.5cm
- Trademark:qinkai
- Origin:China. Heze
- HS Code:9018390000
- Production Capacity:300000pieces,Every Day
Description
Basic Info.
Model NO. qk-11 Transport Package PE Specification 61*38*32.5cm Trademark qinkai Origin China. Heze HS Code 9018390000 Production Capacity 300000pieces/Every DayProduct Description
OverviewQuick DetailsProperties:Injection & Puncture Instrument, Injection & Puncture InstrumentPlace of Origin:Shandong, China
Brand Name:QINKAI
Model Number:CL-IJ0029E
Instrument classification:Class II
Packing:single PE bag
Tube:150cm(PVC)
Spike:Air-vent or non-vent
Drip Chamber:PVC, with filter
Flow filter:PE/ABS
Connector:Luer slip/ Luer lock, Y-type
Needle:injection needle 21G
Certification:ISO13485 CESupply AbilitySupply Ability:
100000 Piece/Pieces per DayPackaging & DeliveryPackaging Details
1pc/PE bag*500/ctn
Port
Qingdao or Shanghai
Best Quality With Disposable Parts Iv Infusion Set With RegulatorDetailed images
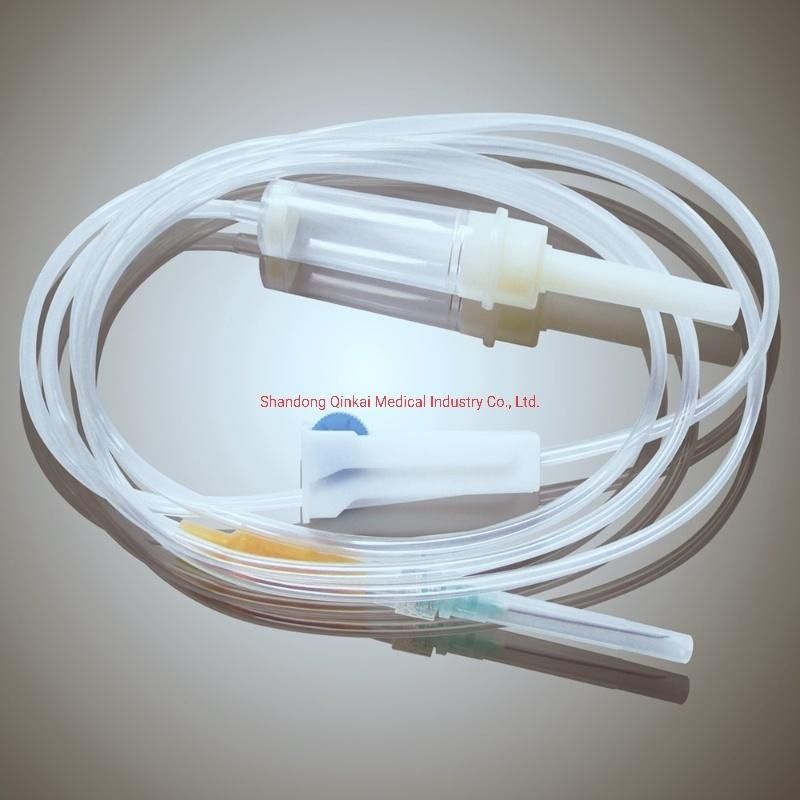
Product Description
Product name: Disposable Infusion Set
Item No.: CL-IJ0029E
Specifications:
Tube: | 150cm(PVC) |
Spike: | Air-vent or non-vent |
Drip Chamber: | PVC, with filter |
Flow filter: | PE/ABS |
Connector: | Luer slip/ Luer lock |
Injection Port: | Latex Injection Port |
Needle: | With Needle( 21G) |
Sterilization: | Sterilized by EO gas, non-toxic, non-pyrogenic |
Scalp Vein Set: | With or without scalp vein set (21G) |
Packaging: | 1pc/PE bag*500/ctn, 65*38*32.5cm3/ctn G.W.:12kg, N.W.:11kg |
Certification | ISO13485 CE |
Main structure, performance
Product consist of Plastic needle, drip bottle, medicine filter, catheter, regulator,ourd latex tube, Screw mouthtwo-way joint, injection needle 21G
Scope of application
Coordinates the disposable use venous transfusion needle, uses in the vein drop for the human body.
Taboo disease, the precautions, warn and briefing explanation
1. Infusion set sterilized by Epoxy hexylene, asepsis,non-pyrogen without unusual toxicity and hemolysis response.
2. This for the clinical disposable use, use after opening.
3. Take down protect set , insert bottle the plug puncture device drains away interior air.
4. Uses the rate-of flow controller to adjust the drop number, according to conventional infusion operation.
5. Stop use if package is punctureed or the protective cap falls off.
6.For single use, damage after use.
7. The term of validity is two years, Expired is forbid.
8. The product should store in the relative humidity not to surpass 80%, non-corrosiveness gas, cool, ventilates good, in dryly clean room.
Advantage of Our Company
1. Professional medical products manufacture for more than 10 years
2. High quality with competitive price
3. Independent Design and Outstanding Sales Team
4. Large Supply Ability
5. Excellent after-Sales service
Factory




Contact person: Jack Jiang
Address:Quancheng road medical device industrial park, chengwu economic development zone, shandong province, China (qinkai group) zip code: 274200
Company Home Page: qinkaiyiliao.en.made-in-china.com
