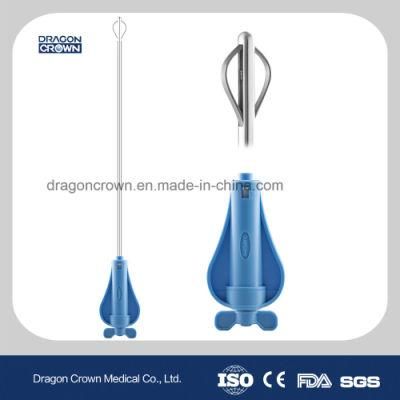
Minimally Invasive Spinal Devices for Vertebroplasy and Kyphoplasty
ZHENGZHOU OHFU INDUSTRY ENTERPRISE CO., LTD.- Type:Surgical Supplies Materials
- Material:Stainless Steel
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:With Logo Printing
Base Info
- Model NO.:202
- Puncure Needle Diameter:2.5mm, 3.0mm, 3.2mm, 4.0mm
- Bone Cement:Italy, Germany
- Transport Package:Sterile Package
- Specification:202
- Trademark:Dragon Crown
- Origin:China
- HS Code:9018909099
Description








Thanks for visiting us!
Please feel free to contact me anytime!
Contact Person: Vivian
Website: dragoncrown.en.made-in-china.com
