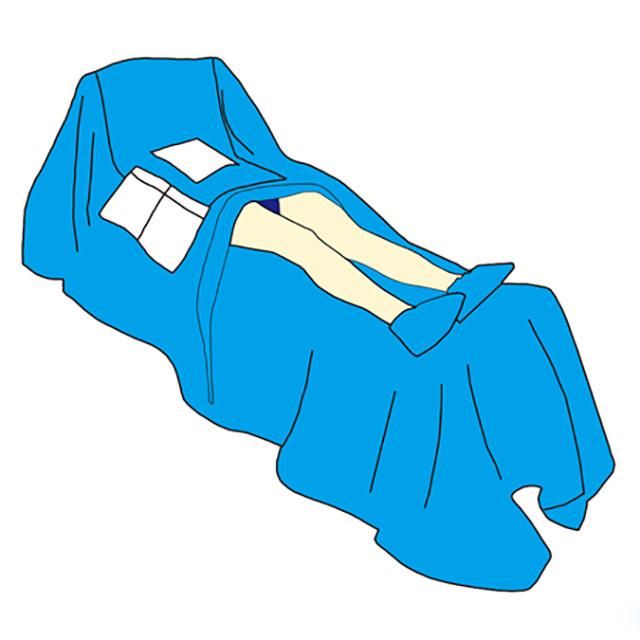
Disposable CE and ISO13485 Approved Sterile by-Pass Surgical Drape
Lanxi Kingway International Trade Co., Ltd.- Material:PP
- Feature:Disposable
- Certification:CE, FDA, ISO13485
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Group:by-Pass Surgery Patients
Base Info
- Model NO.:220*350cm
- Logo Printing:Without Logo Printing
- Transport Package:1PC,Sterile Pouch or 1PC,Paper-Plastic Bag
- Specification:PP, PP +PE, SMS, etc
- Trademark:JOINKONA, OEM
- Origin:China
- HS Code:6307900090
- Production Capacity:50000PCS,Day
Description
Product name
By-pass drape
Measure
Regular size 220*350cm or customized
Material
SMS, Bi-SPP Lamination fabric, Tri-SPP Lamination fabric, Bi-Viscose Lamination fabric, Tri- Viscose Lamination fabric, PE film, SS
Code
JKD-0021 Bi-Viscose Lamination fabric
JKD-0022 Bi-SPP Lamination fabric
JKD-0023 Tri- Viscose Lamination fabric
JKD-0024 Tri-SPP Lamination fabric
JKD-0025 SMS
Label
Feature
Disposable EO(Ethylene Oxide)
Packaging
1pc/sterile pouch or 1pc/paper-plastic bag
Brand
Medical Technology/OEM is available
Description:
By-pass drape is used in sterile cardiovascular operating room surgery. The product is non-irritating, non-toxic, odorless and has no side effects on the human body. Effectively improve surgical efficiency and reduce the risk of cross-infection.
The drape's critical area is surrounded with incise film and long lasting liquid absorbing double-reinforced material, integrating in Carter pockets and cable clamps.