
Disposable Shoe Cover, Non-Skid Shoe Cover, Non-Woven Shoe Cover, Medical Shoe Cover, Medical Non Skid Shoe Cover
Shandong Yusheng Biotechnology Co., Ltd.- Material:Non-woven Fabric
- Color:White,Blue,Green
- Feature:Disposable, Spp20-40G,M2
- Style:Smooth O Plain, Embossed or Textured Surface
- Package:10PCS,Polybag, 1000PCS,CTN
- Usage:Safety & Protective
Base Info
- Model NO.:VSC02
- Model No:Vsc02
- Type:Shoe Cover
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:Without Logo Printing
- Transport Package:Carton
- Specification:40x40cm, 45*40cm, or as required
- Trademark:OEM,VMED
- Origin:China
- HS Code:6307900000
- Production Capacity:100, 000PCS,Day
Description
Specification of Non-skid shoe coverModel NO. VSC02 Size 17*38cm;17*41cm,or as required Material PP30-40G/m2 Packing 100pcs/bag,1000pcs/ctn Surface Smooth or Anti-Skid Surface Usage for Medical, Surgical, Pharmaceutical, Industrial Style Machine-made;Hand-made Origin China Color White/blue/green Certified CE,FDA,ISO
Product Display

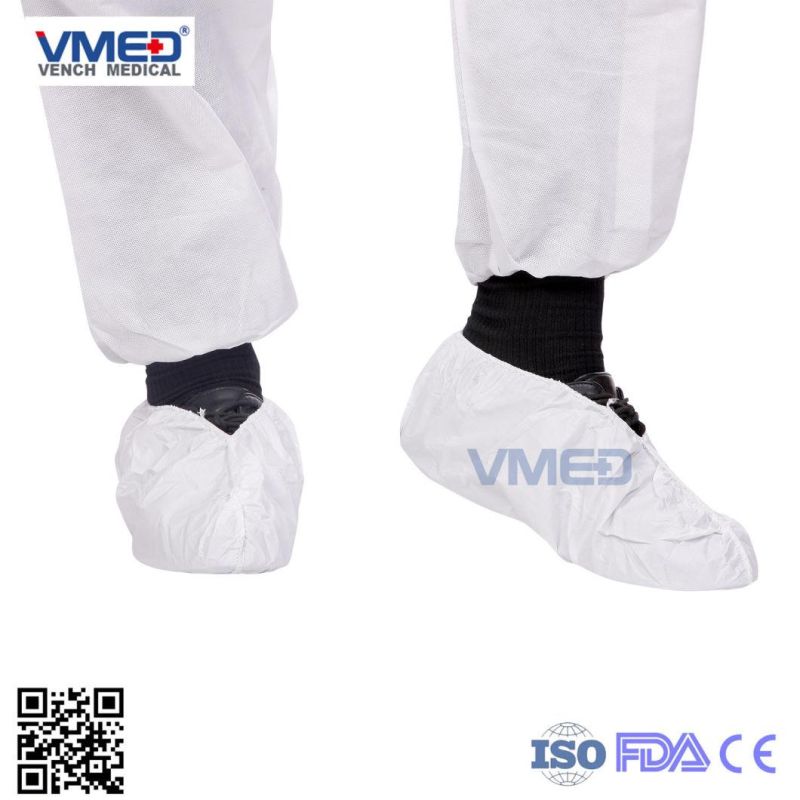





The use of shoe covers
To solve: When guests need to take off the shoes for visits, you need to clean many times in slippers, doormats, the floor and cause you tired
To prevent: private slippers into a public slippers, remove the slippers brought beriberi cross infection spread hidden!
To reduced: constantly cleaning mopping cause the trouble, you can effectively keep the interior clean use the cover!
To improve: change at the shoes cause a variety of bad smell, polluting the indoor environment !
To avoid: when friends and family come to your house make them restraint and embarrassment due to the damaged socks or foot out-door
To put an end to: A lot of visitors , not enough slippers that makes trouble!
Company Information

Let Vench Medical group up with our customers together !
FAQ
Q1: Can I have a sample first ?
VMED:Of course ! Sample are available and free
Q2: Do your company have any quality management system such as ISO, EN or CE certificate(s)?
VMED: Yes, we have CE, ISO13485, FDA,
Q3:What's your advantage? Why we choose you?
VMED: Vench Medical Products Co., Ltd. is a fast growing company, specializing in the manufacture of disposable medical products and personal protection products.
Our company has implemented a strict quality management system (QMS) and has passed ISO13485 certification. Our main products have passed CE certification of European Union (EU) and FDA registration of USA.
Q4 : How does your factory control the quality?
VMED : All manufacturing are absolutely base on the pre-production samples. Meanwhile, we have a complete after-sales system.
Q5: What the expiration date for disposable non-woven products?
VMED: We suggest 2 years of shelf storage
Trust us
-- professional &safety
Tel:+86 514 87770905
Fax:+86 514 87770905
Product Display







The use of shoe covers
To solve: When guests need to take off the shoes for visits, you need to clean many times in slippers, doormats, the floor and cause you tired
To prevent: private slippers into a public slippers, remove the slippers brought beriberi cross infection spread hidden!
To reduced: constantly cleaning mopping cause the trouble, you can effectively keep the interior clean use the cover!
To improve: change at the shoes cause a variety of bad smell, polluting the indoor environment !
To avoid: when friends and family come to your house make them restraint and embarrassment due to the damaged socks or foot out-door
To put an end to: A lot of visitors , not enough slippers that makes trouble!
Company Information

Let Vench Medical group up with our customers together !
FAQ
Q1: Can I have a sample first ?
VMED:Of course ! Sample are available and free
Q2: Do your company have any quality management system such as ISO, EN or CE certificate(s)?
VMED: Yes, we have CE, ISO13485, FDA,
Q3:What's your advantage? Why we choose you?
VMED: Vench Medical Products Co., Ltd. is a fast growing company, specializing in the manufacture of disposable medical products and personal protection products.
Our company has implemented a strict quality management system (QMS) and has passed ISO13485 certification. Our main products have passed CE certification of European Union (EU) and FDA registration of USA.
Q4 : How does your factory control the quality?
VMED : All manufacturing are absolutely base on the pre-production samples. Meanwhile, we have a complete after-sales system.
Q5: What the expiration date for disposable non-woven products?
VMED: We suggest 2 years of shelf storage
Trust us
-- professional &safety

Tel:+86 514 87770905
Fax:+86 514 87770905
