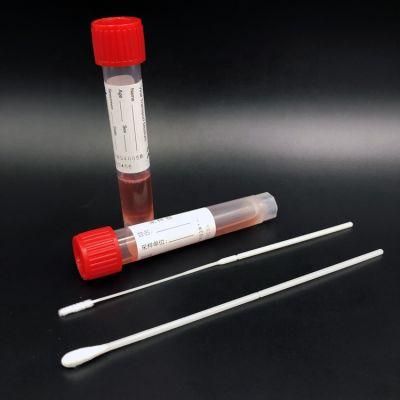
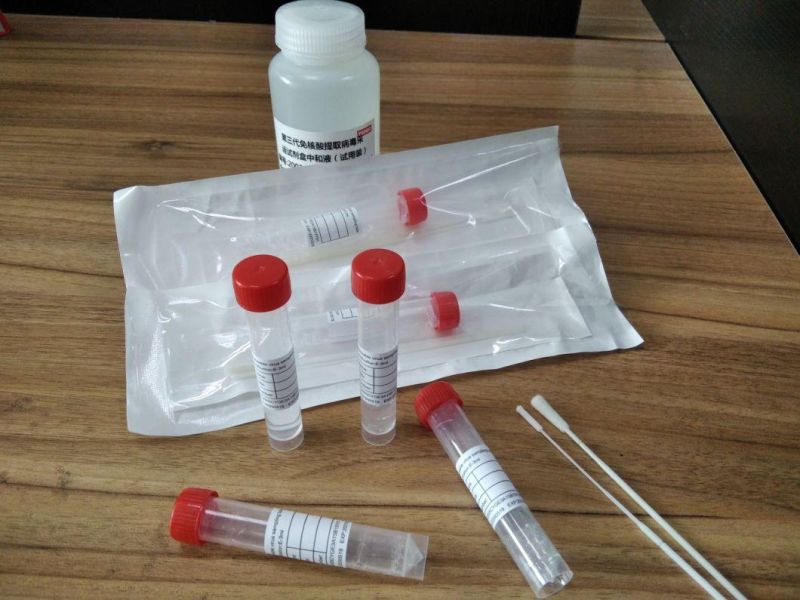
Specimen Collection Device Disposable Virus Sampling Tube/Virus Test Swab
Fushun Bright Science and Technology Co., Ltd.- Type:Surgical Supplies Materials
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:One Year
- Group:Adult
- Logo Printing:Without Logo Printing
Base Info
- Yy/T0287-2003:Yy,T0287-2003
- GB/T19001-2008:GB,T19001-2008
- Transport Package:Packing
- Specification:12
- Trademark:precision
- Origin:Changzhou
- HS Code:9018390000
Description
Basic Info.
Yy/T0287-2003 Yy/T0287-2003 GB/T19001-2008 GB/T19001-2008 Transport Package Packing Specification 12 Trademark precision Origin Changzhou HS Code 9018390000Product Description
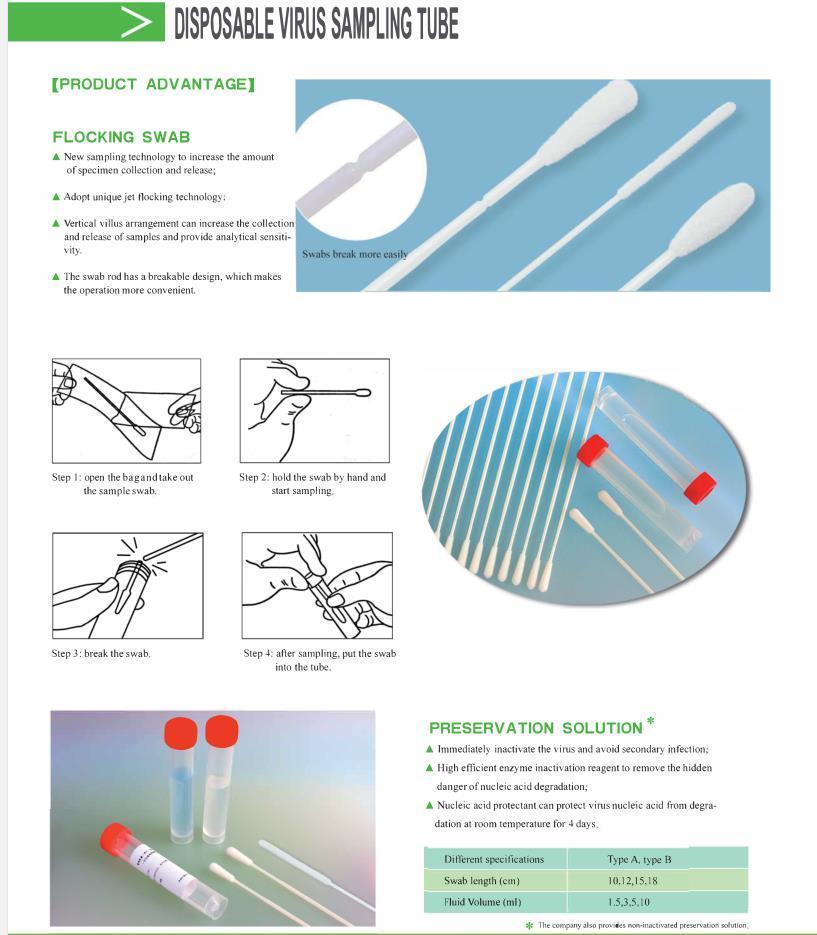
Specifications :| Different specifications : | Type A, type 8 |
| Swab length (cm) : | 10,12,15,18 |
| Fluid Volume (ml) : | 1.5,3,5,10 |
PRODUCT ADVANTAGE
FLOCKING SWAB :
1) New sampling technology to increase the amount of specimen collection and release;
2) Adopt unique jet flocking technology,
3) Vertical villus arrangement can increase the collection and release of samples and provide analytical sensiti- vity.
4) The swab rod has a breakable design, which makes the operation more convenient.
PRESERVATION SOLUTION :
1) Immediately inactivate the virus and avoid secondary infection,
2) High efficient enzyme inactivation reagent to remove the hidden danger of nucleic acid degradation,
3) Nucleic acid protectant can protect virus nucleic acid from degra- dation at room temperature for 4 days

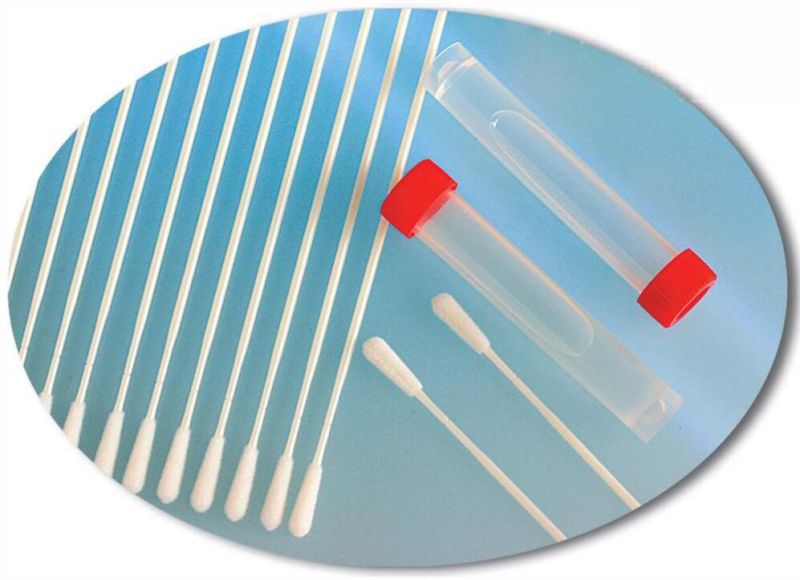



The preservation solution can be divided into three types of inactivated, non-inactivated and non-extracted, which can be selected by customers.
UP +10 YEARS PRO FACTORY
WITH ALL CE /FDA /CHINA CERTIFICATION
5 FLOOR UP 5000SQM PLANT
WELCOMED CHECKING
we can supply you sampling tube and preservation solution and swab
Independent packaging sales
also ok for all types-
inactivated,
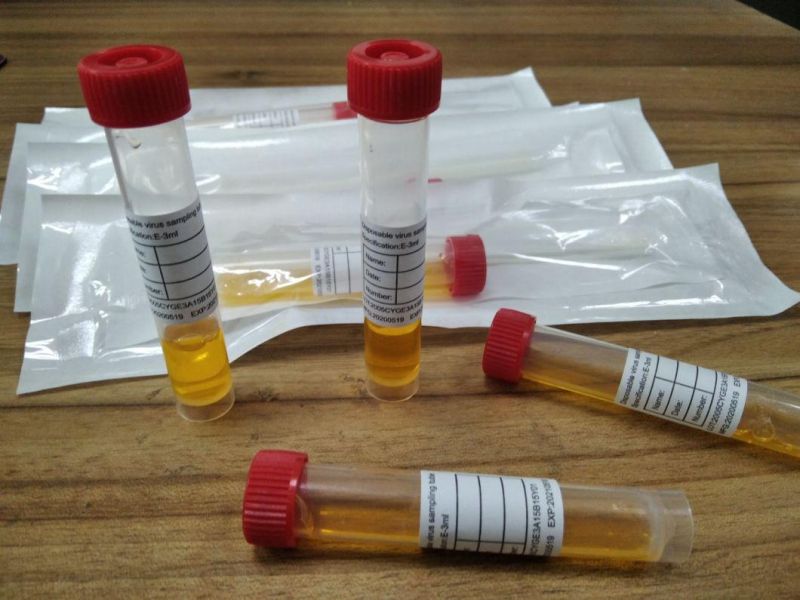
non-inactivated
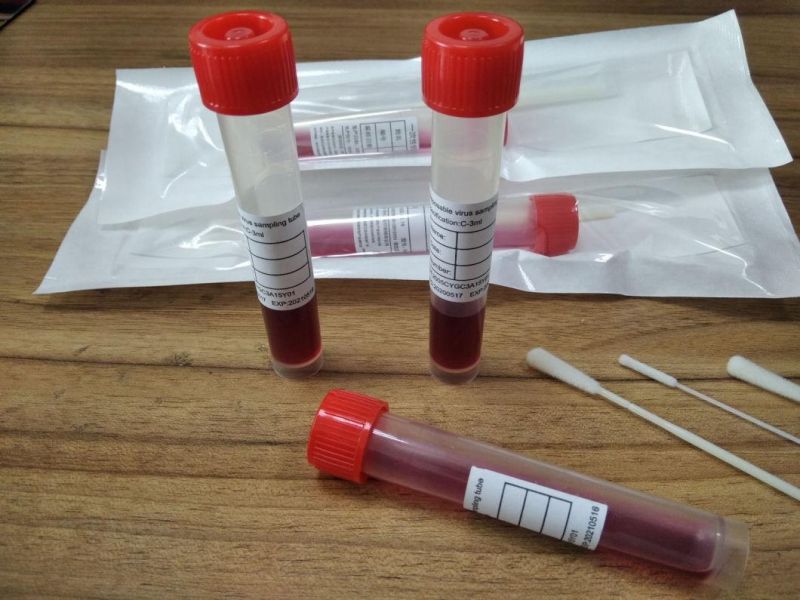
non-extracted
CHINA CERTIFICATION

CE CERTIFICATION


