
2ml Universal Transport Medium Nasopharyngeal Throat Swab Vtm Kit
Guangzhou Wangda Co., Ltd.- Type:Test Strips & Test Tube
- Material:Nylon
- Ethylene Oxide Sterilization:R
- Quality Guarantee Period:One Year
- Group:All
- Logo Printing:With Logo Printing
Base Info
- Model NO.:CY-F005-20
- Transport Package:Indiviual Packaging
- Specification:150mm
- Trademark:iClean
- Origin:China
- HS Code:3005909000
- Production Capacity:800000
Description
Basic Info.
Model NO. CY-F005-20 Transport Package Indiviual Packaging Specification 150mm Trademark iClean Origin China HS Code 3005909000 Production Capacity 800000Product Description
Viral Specimen Collection 2ml Universal Transport MediumNasopharyngeal throat Swab VTM kit


1.Why Cells Specimen Collection by nylon Swab?
Nylon Swabs are ideal for collecting large amount of cells and rapid slution of the specimens instantly release the cells into the cells into the transport medium.
It is well recognized and adopted by the diagnostic test kit manufacturers who produce reagents in molecular genetic,forensics,clinical laboratories sectors.
2.With traditional fiber wound swabs a large percentage of the sample collected remains entrapped in the fiber matrix of the t
It has been shown that the nylon swab releases up to 95% of the sample collected compared to 25% in a standard fiber tipped swab-especially important if the sample size is particulary small.
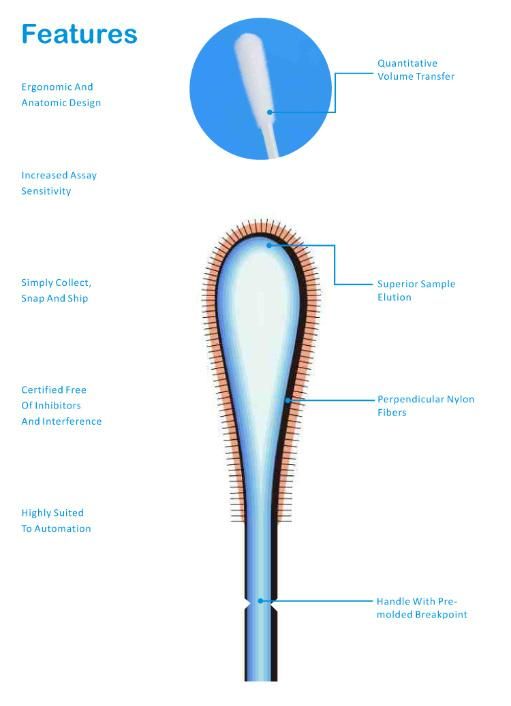
3.The size of swa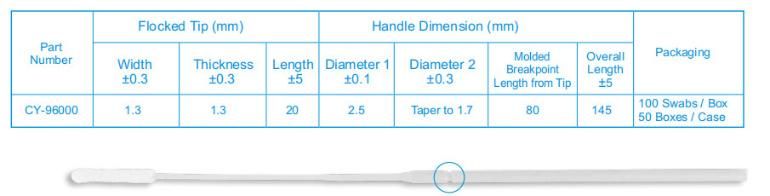
Packaging&Shipping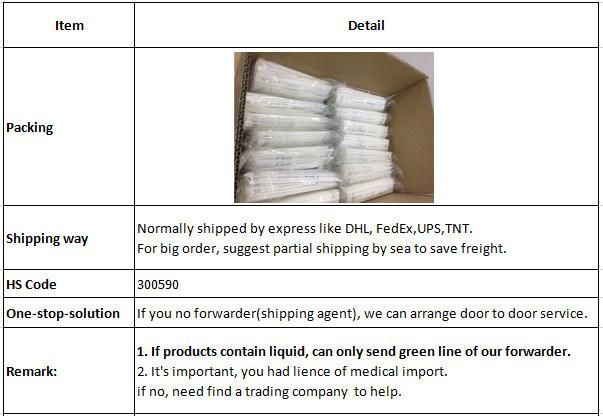
FAQ
1.How many years manufacturer on dna test kit?
Founded on June 2008, we focus on R&D, production and sales of on dna test swab kit.
2.You had patents, certificate of dna test swab kit?
We had more than 22 approved patents on medical consumable & medical service. Now we are China first FDA approved manufacturer from 2014 (DNA collection kit, antiseptic swab), the only one with FDA/CE/Free sale certificate, & GMP factory
3. Your Factory had qualified R&D Lab(dna test swab kit)?
Yes. From 2016, about 56% annual revenue had been funded on the new factory building on genetic test kit , production equipment updating, talent introduction and staff training, construction & management of our new class 100,000 clean room & class 100,00 Laboratory for dna test kit adhere to GMP.
4.Your factory is following GMP for dna test swab kit?
Yes,our new class 100,000 clean room & class 100,00 Laboratory for dna test kit adhere to GMP.
In Jan 2018, Free sale certificate( dna test swab kit) based on GMP is issued to us.
5. OEM / ODM of dna test kit available?
Yes, OEM,ODM of dna test kit are both available.
can customize your logo &text on package & our DNA Test swab collection kit.
6. How you satirize the dna test kit?
Normally by γ ray(Irradiation), EO is also available.
DNA collection swab, antiseptic CHG swab, chg skin prep applicator can be sterile of requested
Company information
Our GMP Factory:
Established in 2008,Huachenyang (Shenzhen) Tech Co., Ltd. is China leading manufacturer on dna test kit. For 10 years,
we're committed to integrating R&D, production & sales of genetic test kit, medical swab and lab reagents.
Our product includes DNA test kit, sample collection swab,skin prep swab applicator and TOC clean validation swab kit,nucleic acid extraction reagent kit, which are mainly used for genetic test, bio-pharmaceuticals, hospitals,clinical diagnostic, microbiology and forensicetc.
In 2016, GMP factory with Class 100,000 clean rooms & 100,000 Lab built;
In Jan. 2018, New Free Sale Certificate issued.


In the air

