-
Bladed Single Use Bladed Trocar 10mm Surgical Instruments

Overview Detailed PhotosProduct ParametersCompany ProfilePackaging & ShippingCertificationsFDA 510K ApprovalFDA 510K Letter: K193272Trade/Device Name: GTK TrocarsRegulation Number: 21 CFR 876.1500Date: July6, 2020CE certificateRegistration No.: HD 60147623 0001Report No.: 1702
-
Gtk Disposable Trocar for Laparoscopic Surgery Bladed Trocars

Overview Detailed PhotosProduct ParametersCompany ProfilePackaging & ShippingCertificationsFDA 510K ApprovalFAQTrocarsQ: Are you a factory or trading company?A: Yes. We are a factory in China with 17 years surgical devices developing and manufacturing experience.Q: What is the
-
Bladed Disposable Bladed Trocar 10mm for Laparoscopic Surgical Instruments

Overview Detailed PhotosProduct ParametersCompany ProfilePackaging & ShippingCertificationsFDA 510K ApprovalFDA 510K Letter: K193272Trade/Device Name: GTK TrocarsRegulation Number: 21 CFR 876.1500Date: July6, 2020CE certificateRegistration No.: HD 60147623 0001Report No.: 1702
-
Single Use Laparoscopic Sugical Trocar Tip Trocar
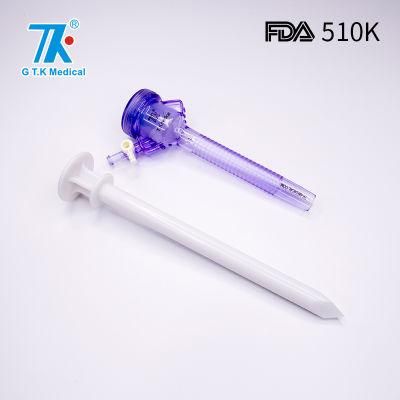
Overview Detailed PhotosProduct ParametersCompany ProfilePackaging & ShippingCertificationsFDA 510K ApprovalFAQTrocarsQ: Are you a factory or trading company?A: Yes. We are a factory in China with 17 years surgical devices developing and manufacturing experience.Q: What is the
-
Disposable Trocar for Laparoscopic Top 3 Manufacturer in China
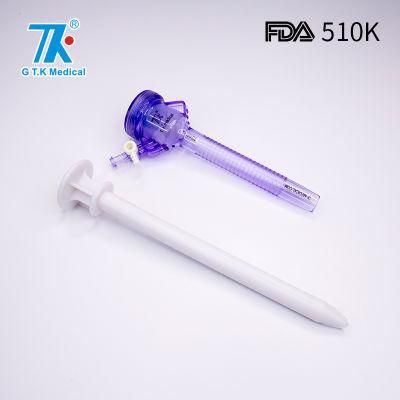
Overview Detailed PhotosProduct ParametersCompany ProfilePackaging & ShippingCertificationsFDA 510K ApprovalFAQTrocarsQ: Are you a factory or trading company?A: Yes. We are a factory in China with 17 years surgical devices developing and manufacturing experience.Q: What is the
-
Sugical Instrument for Gtk Bladed Trocar

Overview Detailed PhotosProduct ParametersCompany ProfilePackaging & ShippingCertificationsFDA 510K ApprovalFAQTrocarsQ: Are you a factory or trading company?A: Yes. We are a factory in China with 17 years surgical devices developing and manufacturing experience.Q: What is the
-
Laparoscopic Disposable Surgical Optical Bladeless Trocars

Overview Detailed PhotosProduct ParametersCompany ProfilePackaging & ShippingCertificationsFDA 510K ApprovalFDA 510K Letter: K193272Trade/Device Name: GTK TrocarsRegulation Number: 21 CFR 876.1500Date: July6, 2020CE certificateRegistration No.: HD 60147623 0001Report No.: 1702
-
Single Use Bladed Trocar 10mm Surgical Instruments

Overview Detailed PhotosProduct ParametersCompany ProfilePackaging & ShippingCertificationsFDA 510K ApprovalFDA 510K Letter: K193272Trade/Device Name: GTK TrocarsRegulation Number: 21 CFR 876.1500Date: July6, 2020CE certificateRegistration No.: HD 60147623 0001Report No.: 1702